GlOBAL AT HEART, LOCAL IN SPIRIT
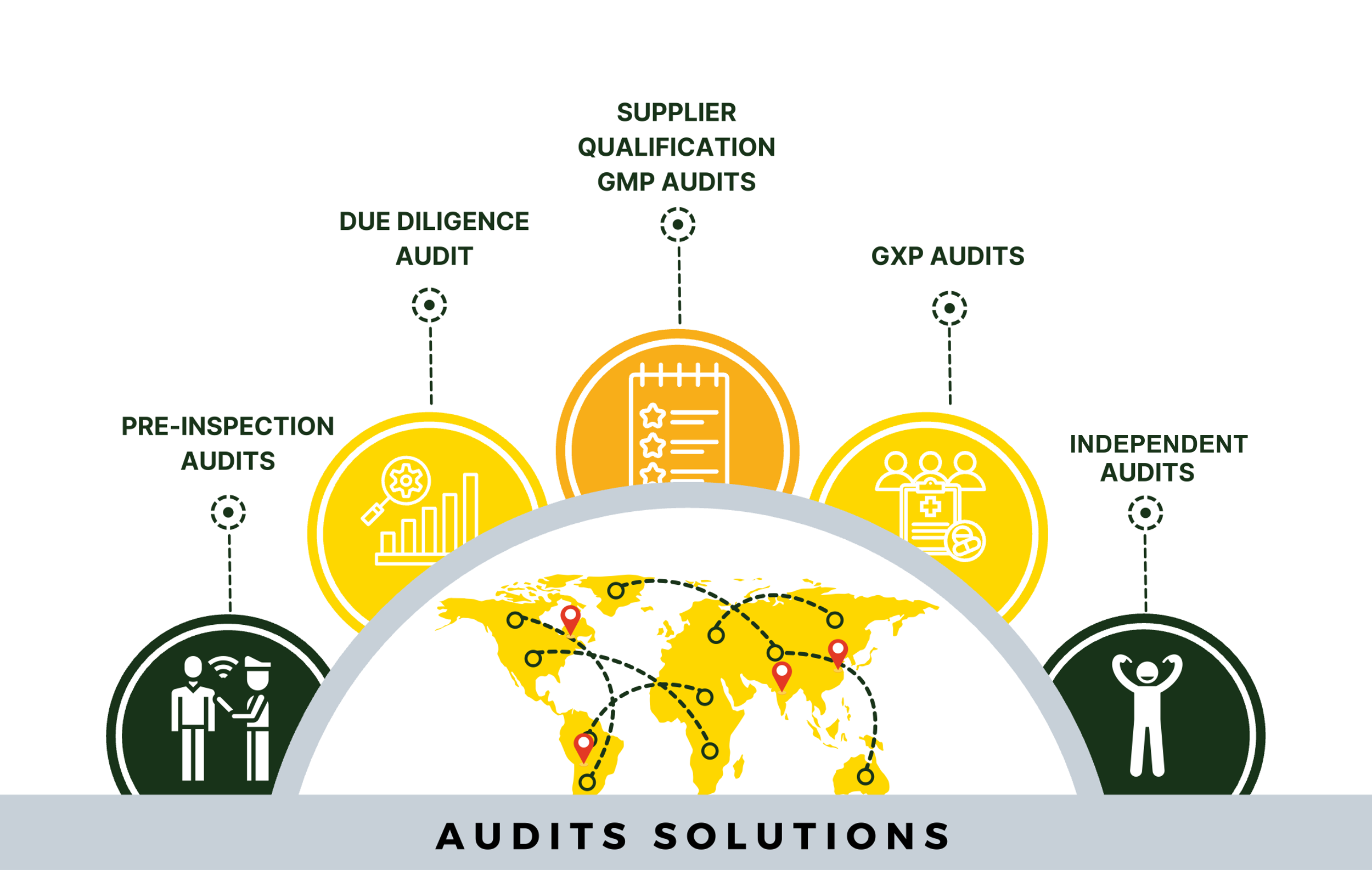
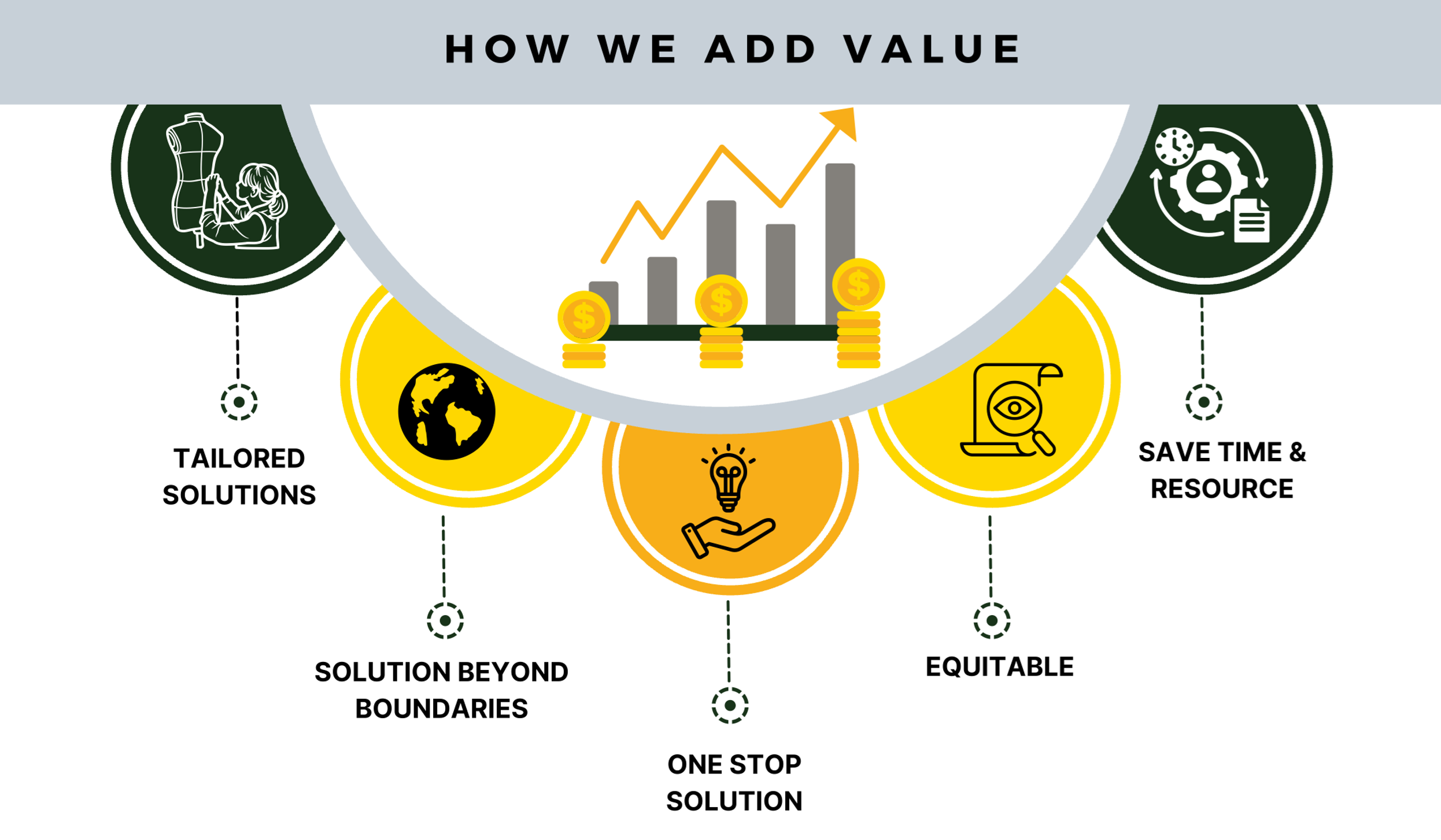
Ideolon offers comprehensive and tailored solutions in supplier auditing with globally present quality compliance consultants who use diverse language skills and technical prowess to ensure quality as well as optimize cost and time.
Ideolon provides comprehensive 360-degree solutions to fulfil GxP requirements, spanning from initial development of drug to its post-marketing phase. The team of more than 70 subject matter experts from 15+ nationalities brings diverse regulatory expertise and years of experience to offer unbiased quality driven solutions.
Audit Approach: Review of Practice to Procedures
At Ideolon, our ideology goes beyond mere review of SOPs. We are committed to ensure compliance of appropriate procedures with a strong emphasis on rigorous practices.
Global Auditing Program- One Point Management
Navigating the global presence of suppliers brings a range of challenges, including language and geographical barriers, as well as reduced operational efficiency and coordination. Ideolon presents unique operational solutions designed to efficiently monitor, enhance, and simplify the auditing process within specified timeframes.